In the context of the current U.S. injection drug use epidemic, targeted public health harm reduction strategies have traditionally focused on overdose prevention and reducing transmission of blood-borne viral infections. Severe bacterial infections (SBI) associated with intravenous drug use have been increasing in frequency in the U.S. over the last decade. This qualitative study aims to identify the risk factors associated with SBI in hospitalized individuals with recent injection drug use.
Qualitative analysis (n = 15) was performed using an in-depth, semi-structured interview of participants admitted to Bellevue Hospital, NYC, with SBI and recent history of injection drug use. Participants were identified through a referral from either the Infectious Diseases or Addition Medicine consultative services. Interviews were transcribed, descriptively coded, and analyzed for key themes.
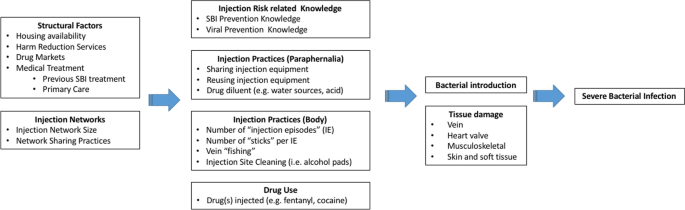
Participants reported a basic understanding of prevention of blood-borne viral transmission but limited understanding of SBI risk. Participants described engagement in high risk injection behaviors prior to hospitalization with SBI. These practices included polysubstance use, repetitive tissue damage, nonsterile drug diluting water and multipurpose use of water container, lack of hand and skin hygiene, re-use of injection equipment, network sharing, and structural factors leading to an unstable drug injection environment. Qualitative analysis led to the proposal of an Ecosocial understanding of SBI risk, detailing the multi-level interplay between individuals and their social and physical environments in producing risk for negative health outcomes.
Structural factors and injection drug use networks directly impact drug use, injection drug use practices, and harm reduction knowledge, ultimately resulting in tissue damage and inoculation of bacteria into the host and subsequent development of SBI. Effective healthcare and community prevention efforts targeted toward reducing risk of bacterial infections could prevent long-term hospitalizations, decrease health care expenditures, and reduce morbidity and mortality.
Bacterial infections due to injection drug use can occur locally at the site of injection or at distant sites through hematogenous spread. Localized infections in people who inject drugs (PWID) involving the skin and soft tissue (cellulitis, subcutaneous abscesses, and venous thrombophlebitis) rarely require hospitalization and are often managed without engagement in medical care [1]. Infections that spread hematogenously and seed distant body sites cause more severe infections, infectious complications, and often require prolonged hospitalizations for intravenous antibiotics and occasional surgical interventions [2, 3]. These severe bacterial infections (SBI) including bacteremia, endocarditis, osteomyelitis and central nervous system abscesses have been increasing in the last decade, mirroring the increase in prevalence of injection drug use [4,5,6,7,8,9,10]. Admission for severe bacterial infections in PWID, specifically endocarditis, is associated with sub-optimal treatment outcomes, high health care costs, and frequent readmission for re-infection [11,12,13,14].
Extensive studies have examined the injection risk behaviors associated with infectious transmission of blood-borne pathogens such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV) [15,16,17,18,19], with interventions reducing drug equipment (syringes, needles, cookers, and cottons) sharing practice proving successful in reducing infection incidence. Bacterial infections have been less well studied. Bacterial skin and soft tissue infections (SSTI) are extremely common in PWID with an estimated annual incidence of 155,000 to 540,000 cases in the United States (U.S.), with the majority of these infections not interfacing with the healthcare system [20]. Despite most cases being managed in the community, SSTI remain one of the most common causes of hospital admission and emergency department visits among PWID [1], with infections caused by resistant bacterial such as methicillin-resistant Staphylococcus aureus seen disproportionately [21,22,23,24].
Prior studies have identified multiple risk factors for bacterial infections in PWID and include injection hygiene, injection frequency, route of administration, anatomic site of injection, polysubstance use—increased risk with opiate-stimulant combinations, as well as length of time injecting, homelessness, gender, sex work, and HIV [25,26,27]. Social-structural determinants of injection-related bacterial infections have been highlighted in the literature, including environmental constraints on the use of sterile water for drug preparation, how providing sachets of acidifier that were too large led directly to over-use of acidifiers and venous sclerosis, and how social and environmental conditions may lead to increased injecting-related skin and soft tissue damage [28,29,30]. Social-ecological models with respect to drug-related risk and harms emphasize the complex interplay between both behavioral and structural factors, with the need for a multi-level approach to harm reduction [31, 32].
Fentanyl has been a less well-studied potential risk factor for severe bacterial infection. Fentanyl-adulterated and/or fentanyl-substituted heroin integrated into the U.S. drug supply in the early 2010s and the vast majority of “heroin” tested positive for fentanyl by the end of the decade [33]. The introduction of synthetic opioids into the US has resulted in a significant increase in U.S. opioid overdose death rates [34]. Fentanyl, with its associated increased injection frequency and high concentration of cutting agents, has been implicated in altering injection drug use practices and subsequent risk of development of SBI [35, 36].
In the context of the current U.S. opioid epidemic, there remains a paucity of research with respect to identifying risk factors for the development of severe bacterial infections in PWID. In the following study, we present qualitative data examining injection and non-injection risk factors associated with bacterial infections among PWID hospitalized with severe bacterial infections.
In this qualitative study, 15 participants (ages 27–57) who self-reported recent injection drug use and admitted to Bellevue Hospital with a SBI were recruited between August 2020 and June 2021 for individual interviews. Bellevue Hospital, the oldest public hospital in the United States, is an 844-bed acute care tertiary hospital located in New York City, New York. Participants were referred to study investigators for eligibility screening from the Infectious Diseases and/or Addiction Medicine inpatient consult services. Eligibility criteria included: reported history of recent injection drug use in the 90 days prior to admission; admitted to an acute care hospital with primary diagnosis of a severe bacterial infection; and ability to comprehend study procedures and provide informed consent. Severe bacterial infections were defined as skin and soft tissue infections requiring surgical intervention (e.g., abscess, necrotizing fasciitis), bacteremia, endocarditis, CNS infections or bone/joint infections (e.g., osteomyelitis, septic arthritis). Each participant was compensated $50 at the conclusion of the interview. All study activities were approved by the Institutional Review Board of New York University Langone Hospital and New York City Health and Hospitals. All participants provided written informed consent prior to being interviewed.
In depth, semi-structured interviews lasted approximately 60 min each. All interviews were conducted on inpatient medical or surgical wards. Interviews were conducted by a member of the research team, including a sociologist and a medical doctor not involved in the treatment of the patient. The interview format was flexible and consisted of open-ended questions that inquired about structural and behavioral domains potentially related to several bacterial infections. Topical domains addressed in the interviews included the following: sociodemographic characteristics, initiation into drug use and specifically injection drug use, current drug use and past trajectories (including patterns of escalation, concurrent or intermittent use of other substances), injection drug use practices, severe bacterial infection knowledge and perceptions of risk, stigma and drug withdrawal during hospitalization, self-treatment and prior hospitalizations related to bacterial infections, as well as structural and psychosocial factors. Interviewees were able to introduce or elaborate on topics of specific relevance to their experience.
Interviews were digitally audio-recorded and transcribed verbatim. Resulting transcripts were descriptively coded and the data analyzed. Themes were identified on the basis of topic and recurrent patterns highlighted throughout analysis of multiple participants’ accounts. Theoretical interpretations resulted from comparative analysis of the most commonly voiced themes and attempted to connect key themes across individual accounts. All participant names have been replaced with pseudonyms. In addition to thematic analysis, key variables were recorded via interview and abstraction of electronic medical record data related to current hospitalization in order to better identify the patient population under study.
The sociodemographic characteristics of the 15 study participants are listed in Table 1. The majority of participants had a primary diagnosis of native valve infective endocarditis (n = 10), with other primary diagnoses including prosthetic valve infective endocarditis (n = 1), spinal epidural abscess (n = 1) and complicated SSTI (n = 3). Thirteen participants had a positive HCV antibody serostatus, of these seven had a positive HCV qualitative RNA during admission. All participants were HIV negative. Six participants (40%) reported at least one prior hospitalization for SBI associated with injection drug use. A causative organism for current hospitalization was identified in thirteen participants and in ten the causative organism was Staphylococcus aureus [methicillin-sensitive Staphylococcus aureus (n = 5), methicillin-resistant Staphylococcus aureus (n = 5)]. A polymicrobial infection was identified in five participants. ICU level care was required in seven participants, mean length of ICU days during admission was 12.3 days (range 2–34). Surgical intervention was required in eleven participants and included mitral valve replacement (n = 3), aortic valve replacement (n = 1), and tricuspid valve vegetation debulking (n = 3). Mean days of parenteral antibiotic administration was 34.3 (SD = 17.8). Nine participants were discharged prior to time of final data analysis and among this group mean hospital length of stay was 33.9 days (SD = 24.9). Four participants left hospital against medical advice during course of treatment and one participant eloped prior to treatment completion and then returned to hospital the following day. Participants’ basic drug-use characteristics are summarized in Table 2.

Our results indicate that multilevel risk factors interact to influence SBI risk. Firstly, we identified structural factors PWID face that could potentiate SBI risk, such as housing availability (i.e., homelessness), harm reduction services (e.g., syringe exchange program access), drug markets (e.g., fentanyl increasing frequency of injection), and medical treatment (e.g., previous SBI treatment could potentially reduce high risk injections, MAT could reduce frequency of drug injection). Secondly, the size of PWID’s injection networks might increase the risk of exposure to bacteria by increasing the number of individuals with whom potentially sharing drug diluting water, syringes and other injection equipment. How often PWID share drugs and injecting equipment within their networks, injection norms behaviors, and frequent engagement in high risk and unsterile practices, will also impact SBI risk. In turn, both structural and injection network variables affect multiple aspects of PWID’s drug use including: (a) knowledge of viral and SBI prevention; (b) paraphernalia-related injection practices (e.g., sharing injection equipment and water used to dissolve drugs); (c) injection practices at the body injection site (e.g., frequency of injection episodes, numbers of injections per injection episode, skin hygiene); and d) which drugs are injected (e.g., fentanyl, heroin, cocaine or a drug combination). The model highlights key aspects related to drug injection practices that may lead to development of SBI: unsafe drug paraphernalia use, which included sharing and reusing injection equipment as well as using non-sterile liquids to dilute drugs, high frequency injection drug use (i.e., increased number of injection events and number of “sticks” per injection event), vein “fishing” (searching the vein after needle puncture), and lack of injection site hygiene (e.g., using alcohol pads to clean injection site prior to puncture). The types of drugs PWID inject could also impact SBI risk, specifically increasing the frequency of injection events, for example, due to the shorter duration of the fentanyl high (versus heroin high) participants reported injecting more frequently throughout a given day.
Structural factors and injection networks have a direct impact on individuals’ injection-related knowledge and injection practices. All together these multilevel factors facilitate the introduction of bacteria into the body, where risk is amplified by repetitive tissue damage caused by repeated injection of drugs and potential injection of particulate matter and harmful compounds. Tissue damage is also inflicted by efforts to self-treat drug-related abscesses. Our analyses indicate that patients hospitalized with SBI have experienced different types tissue damage including: (a) localized (e.g., cutaneous and subcutaneous due to multiple injections, re-use of blunted syringes, and damaging substances including acidic diluents), (b) venous (e.g., repeated penetration leading to scarring, endothelial damage from damaging substances), and (c) distant (e.g., cardiac endothelial damage from particulate matter).
The above findings highlight the complexity of the injection drug use process and the potential social and physiological pathways leading to SBI. This study attempts to understand the multiple domains at the structural, network, and individual level that impact drug injection practices and provide context by which these factors predispose and lead to physiological tissue damage and the development of SBI among PWID. Our proposed Ecosocial understanding of SBI risk adds to pre-existing social-ecological models of drug-related harms by proposing pathways to tissue damage and ultimate development of SBI.
Cumulative damage to the skin and soft tissue at the site of injection increases susceptibility to infection. Tissue damage at sites distant from the injection event, including degenerative changes, areas of prior trauma and valvular endothelial damage associated with injection of particulate matter, likely increase the individuals risk of SBI. Underlying tissue damage at local and distant sites helps create an environment that is favorable for the adherence and proliferation of bacteria, ultimately leading to the development of SBI. High intensity injection drug use is likely associated with increased risk of SBI as Islam et al. demonstrated that reducing injection intensity is associated with decreased risk of invasive bacterial infections among high-frequency injection drug users [27]. Despite the known bacterial infection risk associated with intravenous drug use, few studies have attempted to link the epidemiological and physiological factors associated with SBI in PWID, our study highlights this interaction by the following:
Harm reduction knowledge and psychosocial vulnerabilities influence drug use and high-risk drug injection practices. Perceived likelihood of risk, severity, and susceptibility of SBI among PWID varies widely, and these beliefs can lead to risky injection practices in the context of withdrawal symptoms, drug injection network, and lived experiences. A repeated or temporary lapse in safe injection practices was a recurring theme, often in relation to the severity of addiction, feelings of hopelessness, and vulnerable social situation. Similarly, as reported by others, participants described putting on hold safe injection practices often when “sick” from opioid withdrawal [37]. Those challenging periods undermining PWID willingness to inject safely likely increased opportunities for bacterial introduction during the injection process.
Participants reported high rates of concurrent opioid and cocaine injection drug use (“speedball”). Fentanyl and cocaine were both reported to increase the frequency of injection. High frequency drug use is likely associated with increased cumulative tissue damage and multiplies the number of opportunities for bacterial introduction . Furthermore, the short half-life of these substances may precipitate more frequent withdrawal episodes and therefore, as indicated above, lead to increased frequency of unsafe injection practices. MIPIE, repeated injection at a single body site, vein “fishing,” and use of larger gauge needles cause cumulative damage to skin and soft tissue that may increase SBI risk. MIPIE has been reported to increase the risk of HIV and HCV infections [37]. Increased risk of bacterial infections with “speedball” and other opiate-stimulant combinations has also been reported in the literature [38, 39]. In addition, cocaine may have a greater SBI risk compared to other street drugs as it can induce constriction of blood vessels, resulting in tissue damage secondary to inadequate blood flow, and may have greater propensity to cause injury to the myocardial surface [40, 41]. Additionally, crack cocaine is not readily soluble in water and is typically prepared using an organic acid, potentially furthering tissue damage and providing a favorable environment for bacteria [42]. One participant reported frequent injections of crack cocaine and over time this led to extreme difficulty finding a usable vein. Concurrent drug use in the same injection increases the likelihood of multiple different cutting agents being present, which may increase the odds of particulate matter entering the bloodstream and damaging the valvular endothelial cells. We posit that this repeated tissue damage both locally and at distant sites increases the risk of SBI among PWID. This direct impact of these external factors on and in the bodies of PWID is consistent with the notion of embodiment brought forward by Krieger. Embodiment refers to how humans literally incorporate, biologically, the world in which we live, including our societal and ecological circumstances [43, 44].
Lack of appropriate hygiene, storing of used (contaminated) syringes, re-using and sharing equipment, use of non-sterile drug diluting fluid, can provide opportunities for introduction of bacteria during the injection process. Socioeconomically disadvantaged participants frequently reported difficulties finding sterile water to use as a drug diluent and would often use discarded or re-used bottles as a source of water, increasing the risk of oral flora contamination of these water sources. Failure to adequately perform skin hygiene prior to injection and subsequent re-use of the needle could lead to the contamination of the syringe with skin flora. Storage of cottons/filters and cookers already exposed to wet material could provide an environment for bacteria to remain viable and proliferate. The re-use of this drug injection equipment not only exposes the PWID to bacteria [45] but potentially a higher inoculum than other possible pathways for bacterial introduction. This study indicates the need for widespread provision of harm reduction supplies to PWID including clean injecting equipment and sterile water.
Self-treatment of abscesses by PWID is common and may lead to the development of SBI. Localized SSTI as a result of injection practices was a recurring theme and perceived to be manageable without seeking medical attention. Perceptions regarding risk, anecdotal experience with self-treatment, as well as physical factors including one’s addiction may explain reluctance to seek medical care. Additionally, prior negative experiences within the context of inadequate opioid withdrawal management may also lead to reluctance to seek medical treatment [46,47,48].
Practice guidelines for uncomplicated skin abscesses following incision and drainage recommend antibiotic therapy to decrease the risk of infection [49]. Hygienic conditions and therapy are not easily available to the PWID who self-treat. Inadequate treatment and persistence of infection may precipitate hematogenous spread. Underlying tissue damage both locally and at distant sites would allow for adherence and propagation of bacteria. Increased medical management of uncomplicated SSTI among PWID would likely decrease rates of treatment failure and risk of progression to SBI. For participants with prior SBI hospitalization, adaptation of safer injection practices as a result of education and past experience were reported to occur yet did not lead to prevention of future SBI in this population. This may be related to limited knowledge and omission by healthcare providers surrounding the multiple potential amplifiers of bacterial infection risk during the injection process.
PWID admitted to hospital with SBI should be treated in a multidisciplinary manner with particular focus on avoidance of withdrawal symptoms to limit failure to complete treatment and potential high-risk behaviors while hospitalized. Participants reported using intravenous drugs while hospitalized and noted unsafe injection practices (e.g., needle re-use, using medically placed venous catheters) while doing so. Managing withdrawal symptoms in hospitalized PWID with SBI would ultimately decrease risky injection practices that may lead to severe complications, elopement or leaving the hospital against medical advice, as well as ensuring completion of treatment course [50].
The evolving opioid epidemic coupled with limited knowledge of potential risk factors and increasing incidence of SBI in PWID, provides a significant opportunity for intervention that may reduce morbidity and mortality in this vulnerable population. Harm reduction strategies targeting SBI will need to be comprehensive given multiple potential means of introducing bacteria into the process and fluid nature of the risk (during and between separate injection events). The typical medical professional offers minimal information (i.e., clean needle use, avoid needle sharing) for safe injection in the context of complex and varied behaviors. PWID interviewed in this study demonstrate what is likely widespread basic understanding of safe injection practices. In addition, PWID interviewed noted information about “safe” practices often travels via word of mouth rather than from medical professionals. PWID would benefit from pervasive messaging throughout the medical system, provided through a more complex and in depth understanding of the potential risks and prevention strategies.
Harm reduction messaging consistently acknowledges the importance of access to clean injection equipment in the prevention of blood-borne viral pathogens such as HIV and hepatitis C, however guidance on the prevention of bacterial infection is limited [51, 52]. Amending current harm reduction messaging is likely to be important, especially in the context of growing acceptance of observed consumptions sites [53], and potential opportunity at these facilities to identify PWID that may be at higher risk for bacterial infections as a result of their underlying tissue damage or injection practices. Interventions could include earlier medical evaluation for skin and soft tissue infection and training to improve sterile, and less risky, injection practices. Lastly, beyond injection supplies and prevention knowledge, the embodiment of harsh socio-ecological factors leading to SBIs (and other infections), calls for a forward acknowledgement and welcoming of PWID’s bodies in harm reduction services. As such, availability of food for those who are hungry, a place to rest for those who are tired, a nurse to attend to sickness and abscesses, clean clothing, and showers for those who cannot have access to them would go a long way in facilitating the hygienic conditions SBI prevention requires.
This study is limited by a small sample size of 15, predominantly male, White, and older than what may be representative of other PWID populations within NYC or the U.S. Potential bias in participant responses could have occurred given participants were recruited and interviewed in a medical setting, and a member of the research team conducting the interviews included a medical doctor. Convenient recruitment of participants may introduce bias and limits generalizability of the results. Future work should aim to validate our proposed theory with larger samples and increased diversity within the participants.
Structural factors and injection drug use networks directly impact drug use, injection drug use practices, and harm reduction knowledge, ultimately resulting in tissue damage and inoculation of bacteria into the host and subsequent development of SBI. Despite perceived safe injection practices among PWID, limited practice of these behaviors and knowledge deficit on how to reduce their risk of drug-injection-related SBI was common. Effective healthcare and community prevention efforts targeted toward reducing risk of bacterial infections could prevent long-term hospitalizations, decrease health care expenditures, and reduce morbidity and mortality.
The datasets generated during and/or analyzed during the current study are not publicly available due to them containing information that could compromise research participant privacy/consent but are available from the corresponding author on reasonable request.